It might seem an obvious thing to recognize, but most organizations strive to produce a product that meets it’s customers’ requirements (some you may be surprised to find out even try to excel them!)
While this may seem self-evident (after all, who looks to provide bad products?), for many, meeting requirements equals compliance against regulations. These can be mandatory within the business sector(such as regulatory conditions like ISO 13485) and enable the business to be carried out in the first place.
For example, if you’re in the aerospace parts business, complying with AS9100 is a requisite allowing you to trade with aerospace manufacturers.
Organizations typically utilize systems to ensure compliance to requirements; we will be taking a look at one such system, process validation, as the topic for this article
What is Process validation
The goal of process validation isn’t as simple as ensuring that your end products are to a high standard. Effective process validation is not guaranteed by product inspection of an end product alone.
Instead, each step of your manufacturing process requires control. Driving compliance in order that your finished product meets all critical elements of requirements and standards.
Additionally, effective process validation has to be based around objective information and analysis, which means investing in data gathering and analysis methods and systems.
In order to facilitate this, a business needs to deploy a system that helps you control the quality of your output, ensuring processes (from design through to manufacture) are optimized in order to meet and sustain the standards required.
Process validation definition
The resultant process validation can be defined as:
“A formal methodology that facilitates product manufacture utilizing defined process and material boundaries resulting in a product(s) that consistently meet defined specifications and requirements.”
What is the difference between Process Verification vs Validation
In assessing an organisations capability, you’ll likely find that most organizations have processes in place to evaluate finished products, these usually apply a level of quality department resource to inspect products produced either internally or via 3rd party suppliers.
This type of inspection typically takes place at the end of the manufacturing cycle and usually involves assessing parts & documentation available at this stage.
As we described above, one cannot ascertain quality by merely testing a finished product. Quality has to be built in via the design & manufacturing process.
Process validation relies on a level of stability; for example, the process steps, tolerances applied, tools & equipment utilized are all standard from one production run to another. Coupled with this, it entails the gathering and analysis of data, obtained during the design and manufacture stages of a product lifecycle, to ensure that the process can consistently deliver products to the required standard.
Typical steps of process validation
While there are several models for process validation (based around product and industry), some industries mandate the activity. An example of this is within pharmaceuticals. As a result, in these sectors, processes and methods are typically mature and provide valuable insight into how they can be applied elsewhere, so we’ll be using this model as a good example to help us articulate the process.
Typical process validation is divided into 3 core stages.
A) Process design – Where the manufacturing process has been designed around knowledge of the product and customer requirements
This stage would typically include attributes such as:
- Design – taking into account requirements & likely production methods.
- Identification of Key requirements,
- Critical Process Parameters and
- Critical Quality Attributes
- Statistical techniques to determine the probability of successful manufacture
- Utilising models & tools such as Design for experiment to validate the design (i.e. by investigating tolerances vs likely Production Methods). Design of experiments can be used to identify features that require specific controls in order that they can be adapted and built into processes.
- Prototyping
- Optimization
This information is then utilized to design the manufacturing process & appropriate controls. This ensures that the entire manufacturing process will meet the defined requirement on a consistent basis.
b)Process Qualification
Demonstrating and then qualifying the processes designed at earlier stages.
The organization will look to ensure that product attributes have been effectively controlled through a robust design phase.
C) Process verification
An effective monitoring solution that includes verification and validation of critical attributes providing assessment and assurance gained throughout the production process.
Process validation in practice
While you often see process validation utilized in a manufacturing sense, process validation can (and is) used to assess a range of processes through the product life cycle from design and development through to Sales and disposal.
By undertaking process validation, you should be utilizing objective analysis and evidence to determine that a product meets the requirements of it’s intended use and further meets its specific parameters.
The key to controlling these parameters it to understand the acceptable range/tolerance that an operation can maintain. These may range from organization to organization and industry to industry but the fundamental basics remain the same, which is to determine the operating envelope that will consistently produce an acceptable product.
As you’ve read above – objective evidence means data. Therefore a key up front element of the process is to consider the methods for both data capture and also its analysis.
During this piece, we’ve predominantly discussed validation ahead of serial production of a product but there are some other flavors:
- Prospective validation – The execution of process validation prior to process deployment
- Retrospection validation – validating processes that have been already implemented utilizing historical evidence
- Concurrent validation – Validating processes during implementation of a process
- Revalidation – Revalidating processes, previously determined as fit for purpose.
Process validation example
In our simple example we’ll take a look at the process of making a cup of tea.
The customer requirement is a hot, sweet beverage.
Let’s start out with the basics.
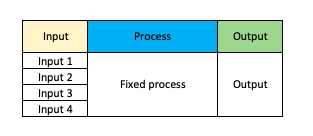
As we can see in the below picture we have
- A series of inputs
- A fixed process
- An output
Now our output is subject to some variables that we’ll need to control so we’ll expand out the initial picture to the following:
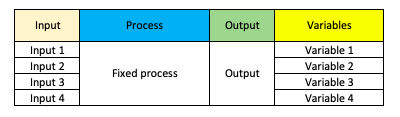
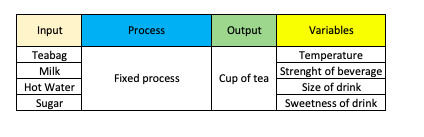
During the product design phase, we quantified our output variables around 4 product attributes:
- Temperature
- Strenght of beverage
- Size of drink
- Sweetness of drink
Now when we look at our simple process, we are able to review:
- The method of measurement/Analysis we’ll use to validate each step (i.e. Boiling water can be quantified with thermometer),
- The acceptable tolerance that can be applied – in the case of the boiling water out lowest acceptable limit is 98 degrees and our upper one is 102 degrees.
As we described above, I would have expected that our tolerances have been set during our design phase, where experiments would have been run to test tolerances and thier impact on end-product ensuring that we gained quantitative insight into what was acceptable and what was not.
This would’ve been done with an understanding of the capabilities of the manufacturing process.
The process steps can be seen below
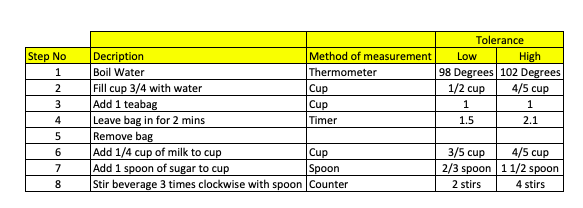
As you can see by simply assessing the output at the end of the process, we may well end up with a sweet cup of tea but importantly, it may not meet the design requirements (i.e someone may put 3 teaspoons of sugar). The validation steps within our process ensure that we have a product that meets both our design requirements + the customer needs.
As you can see from the above example, Process validation takes the process of quantitative and qualitative assessment of products several stages further and is a continual process requiring constant review and development, to ensure that it meets the requirements of the business.
With the absence of suitable and operationally competent processes to drive these standards, the consequences can be disastrous. Not only can products be produced that do not meet requirements, but should you fail to meet a legislated standard, then you may not be able to do business in the first place.
Benefits & issues when utilizing process validation
As we’ve described above, there are two key drivers for utilizing process validation – the first is meeting the requirements of your customer, and the second is industry-specific legislative requirements.
There are numerous other benefits, however, which include:
- Demonstrating that products produced meet the customer requirements
- Demonstrate that processes are controlled (this might include manufacturing processes, processes around the maintenance of tools and equipment, Supply chain etc)
- Demonstrate consistency & maintain quality
- Consistent validation of process
- Upfront identification of compliance vs non-conformance
- Obvious links with Continuous process improvement
- Support compliance with standards
- Demonstrate control over production
- Increase Yield
- Reduce costs (which include reducing scrap or non-conformance)
- Drive high levels of customer satisfaction
Ultimately process validation confirms that the processes that you have drives the intended results (because you have assessed the data that tells you this).
As with all methods, however, there are some issues that require avoidance these include:
- Fuzzy product requirements (lack of clarity around customer needs/specifics)
- Results lean towards verification rather than validation – (e.g certain steps maybe impossible to validate after final assembly, investigating the final assembly may not validate the process (e.g were components assy’d correctly).
- Lack of organizational knowledge of process steps like design for experiment
- Poor quality data is used within the process
- Lack of knowledge of 3rd party supplier capability (or failure to reach out and ask)
- Design phase divorced from production element and unwillingness to collaborate
- Failure to build lessons learned into product
- Failure to utilize process validation as an ongoing process with the belief that it is just done once
This final point is perhaps the crucial one to end on. Consider these likely changes to a business:
- Production is relocated
- Additional features added to the product
- Production run is dramatically increased or reduced
- Non-conformances observed.
- New technology introduced in production
- 3rd party supplier changed for an alternative.
The above simple examples illustrate perfectly why process validation must be considered an ongoing process.
Summary
We hope you enjoyed this article on process validation. If you utilize process validation in your workplace and have ideas and suggestions you’d like to share with our readership, then we’d love your feedback. You can message us on Twitter or leave a comment in the section below.
